Site: Zama Field
Lat/Long:
59.07 -118.88
Why This Location
In Phase II of the Plains CO2 Reduction (PCOR) Partnership Program, the project brought together Apache Canada Ltd. and a number of other industry partners to study carbon dioxide (CO₂) capture; transportation; and monitoring, verification, and accounting (MVA) within Apache’s acid gas (sulfur dioxide [SO2], CO₂, and hydrogen sulfide [H₂S]) enhanced oil recovery (EOR) and CO₂ storage project at the Zama Keg River F Pool in northern Alberta. The objective was to build the technical expertise required to facilitate future large-scale carbon capture and storage (CCS) projects and validate techniques for MVA of stored CO₂ volumes. The validation study was designed to: (1) determine CO₂ and/or H₂S vertical migration, or lack thereof, from the pinnacle; (2) develop reliable predictions regarding the long-term fate of injected acid gas; and (3) generate datasets that will support the development and monetization of carbon credits associated with the geologic storage of CO₂ at the Zama oil field.
Research Q&As Discovered
Are the pinnacles of the Zama oil field sufficient to store acid gas and prevent vertical migration? Geochemical modeling and laboratory rock analysis investigated the reactions between Zama-type acid gas and typical Zama reservoir rocks. Model results indicated that the reactions between the acid gas and reservoir rocks can lead to varying degrees of dissolution and precipitation. Laboratory analyses examined geochemical interactions between reservoir rocks, brine, pure carbon dioxide (CO₂), and CO₂–hydrogen sulfide (H₂S) under Zama reservoir pressure and temperature conditions. The results showed no clear differences in pre- and post-exposure mineralogy; however, a clear decrease in the reactivity of both calcium and sulfate was observed in samples exposed to the H₂S gas stream. Further, total dissolved solids data indicate that CO₂–H₂S dissolved less total mineral content, which will presumably correspond to minimal loss of structural integrity of the storage formation.
The effects that acid gas streams may have on wellbore cement were examined by exposing cement cores to pure CO₂ and CO₂–H₂S mixtures under Zama reservoir conditions. The addition of H₂S to the system resulted in the formation of ettringite throughout the cement and precipitation of pyrite in the carbonate rim, which can potentially degrade cement integrity. However, results also indicated that CO₂ in the system may dissolve the ettringite and reprecipitate calcium carbonates that may help improve overall cement integrity. These observations are supported by the fact that industrial-scale acid gas injection projects have been conducted in Alberta for more than 20 years with no reported breaches in wellbore integrity of acid gas injection wells.
Lessons Learned that Impacted Phase III Design?
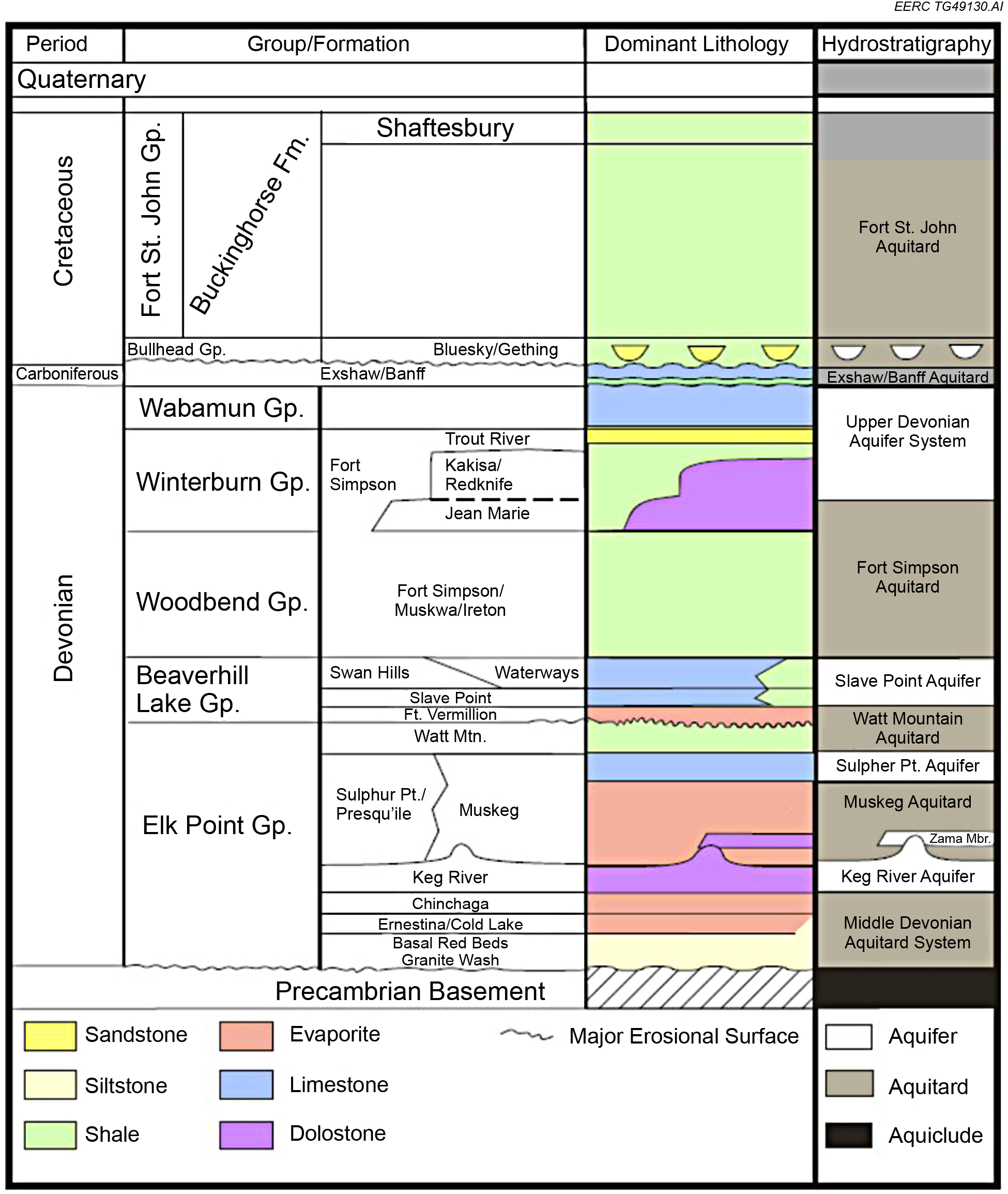
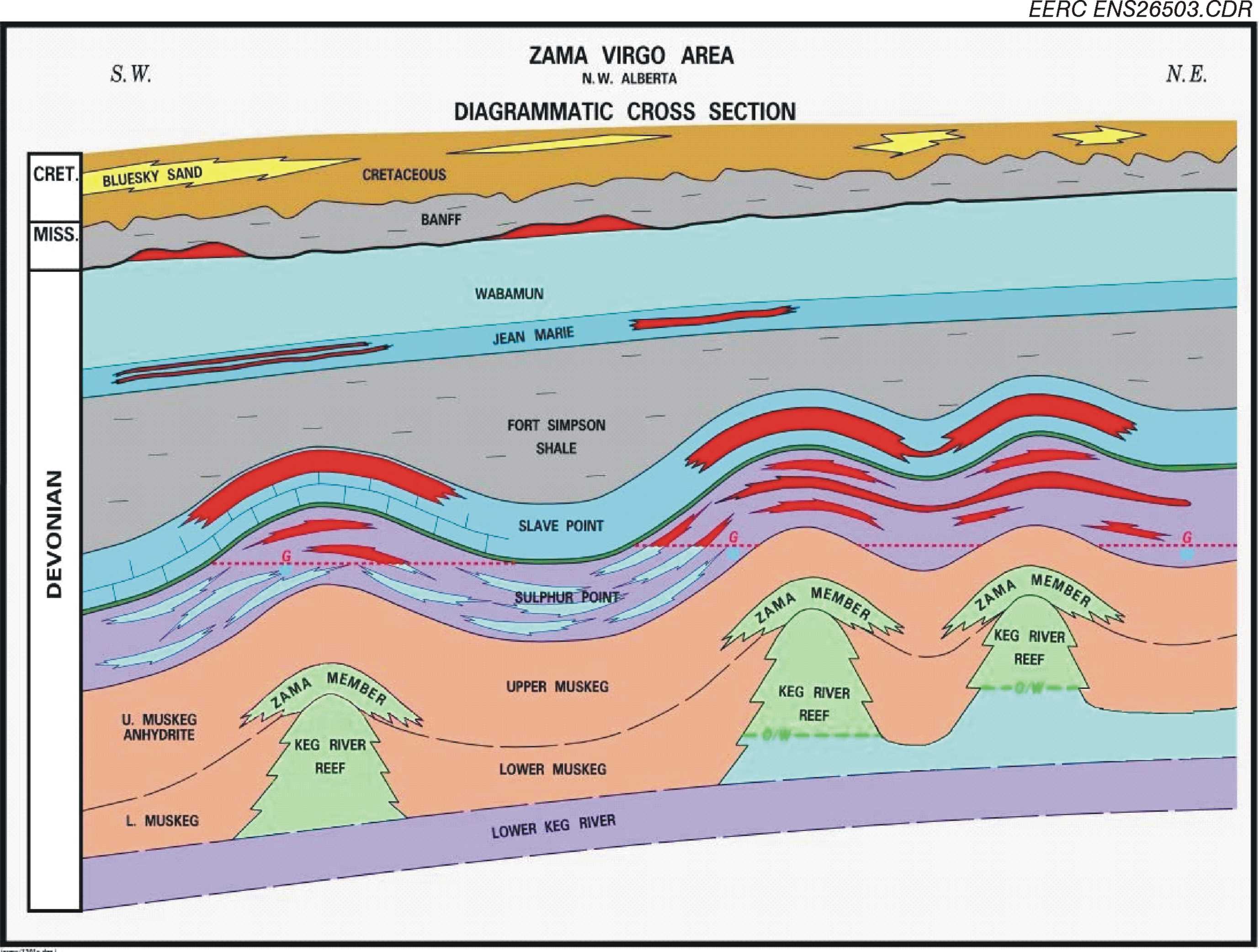
The Zama project investigated geologic characterization, monitoring, and geochemical and geomechanical modeling of carbon dioxide (CO2) storage at an enhanced oil recovery (EOR) site. Characterization work involved investigating the near-wellbore, reservoir, and basin-scale geologic framework with the intent of understanding the long-term storage and transport mechanisms for injected acid gas (CO2 + hydrogen sulfide [H2S]). Site monitoring consisted of fluid sampling, pressure monitoring, investigation of wellbore integrity, and tracer studies within the reservoir and overlying formations. Geochemical laboratory experiments and modeling determined the potential for reactivity of the carbonate reservoir, reservoir fluids, and overlying anhydrite caprock in the presence of acid gas. Mechanical integrity of the injection zone was determined through geomechanical testing of reservoir and caprocks and was simulated to understand the impact of fluid injection and withdrawal within a closed-loop system. The learnings through these activities directly informed Phase III project planning as similar techniques were implemented and expanded upon in future Plains CO2 Reduction (PCOR) Partnership projects.
Story of Interest
Large piles of yellow material are prominent at many oil and gas field operations. This is elemental sulfur, created from the treatment of acid gas. Sulfur has a wide variety of manufacturing applications, including in the pharmaceutical, agricultural, and cosmetic industries. However, production of sulfur often exceeds market demand, and remote locations like Zama have significant transport costs, adding to the challenge of selling this byproduct.
Acid gas is present in many oil reservoirs, typically comprising a mixture of carbon dioxide (CO2) and hydrogen sulfide (H2S). Unlike inert CO2, H2S is extremely hazardous to human health and must be treated (creating the sulfur byproduct) or flared to minimize risks; the windsocks commonly seen at oil field operations allow personnel to identify safe, upwind locations in the unlikely event of an H2S leak.
Enhanced oil recovery (EOR) offers an attractive alternative to costly treatment and stockpiling of sulfur. The closed-loop system used for recycling injected gases minimizes risks associated with handling H2S, avoids costly treatment and stockpiling requirements, improves oil recovery, and stores CO2. Long-serving University of North Dakota Energy & Environmental Research Center (EERC) geologist, Steve Smith, led the Plains CO2 Reduction (PCOR) Partnership field efforts at this remote location and stated, “Our assessment of associated storage at Zama added value by providing extra confidence to the oil company that using produced acid gas for EOR would improve the economics of the overall operation.”
Geologist Rock-Fest
The Keg River reefs provide high-quality oil production and storage reservoirs as they contain a high percentage of good to very good permeability. Zama oil production primarily originates from within the Middle Devonian Keg River pinnacle reef reservoirs at a depth of approximately 1,494 meters (~4,900 feet). The pinnacle reef buildups were formed in a lagoon partially surrounded by carbonate banks and fronted by the Presqu’ile Barrier to the west. To date, more than 600 pinnacles have been discovered in the Zama subbasin. The pinnacle reservoirs typically consist of dolomite with highly variable porosity and permeability and are surrounded and overlain by very tight anhydrite Muskeg Formation caprock, typically 60 to 90 meters (200 to 300 feet) thick.
Links to Phase II EDX Data/Documents
Zama Field Validation Test Regional Technology Implementation Plan
https://edx.netl.doe.gov/dataset/pcor-partnership-phase-ii-zama-field-validation-test-regional-technology-implementation-plan
Regional Technology Implementation Plan for the Zama Field Validation Test.
Zama Field Demonstration Site – Field Validation Test Design Package
https://edx.netl.doe.gov/dataset/plains-CO2-reduction-pcor-partnership-phase-ii-zama-field-demonstration-site
The field validation test and design package of the Zama Field demonstration site.
Field Validation Test at Zama, Alberta – Outreach Action Plan
https://edx.netl.doe.gov/dataset/field-validation-test-at-zama-alberta
Draft Outreach Action Plan for the Field Validation Test at Zama, Alberta.
Field Validation Test at Zama, Alberta – Sampling Protocol
https://edx.netl.doe.gov/dataset/field-validation-at-zama-alberta-sampling-protocol
Sampling protocol for the Field Validation Test at Zama Field.
Zama Field Validation Test – Site Health and Safety Plan
https://edx.netl.doe.gov/dataset/zama-field-validation-test-site-health-and-safety-plan
Site health and safety plan for the Zama Field Validation Test.