Isolated Single Metal Atoms Supported on Silica for One-Step Non-Oxidative Methane Upgrading to Hydrogen and Value-Added Hydrocarbons
Last Reviewed Dated
03/14/2024
Goal
This research aims to create novel, resilient, inexpensive, active, and selective catalyst materials to concurrently conquer current constraints and achieve an efficient, scalable, and intensified non-oxidative methane conversion (NMC).
Performer(s)
University of Maryland, College Park, MD 20742
Background
The catalysts proposed in this project are made of isolated single metal atoms supported in a silica matrix and operated at medium-high temperatures (900-1100 °C). The isolation of metal atoms achieves methane activation by heterogeneous surface dehydrogenation to generate a hydrocarbon pool and hydrogen species, followed by C-C coupling on the active sites, and limits coke formation due to the absence of metal atom ensembles. The high reaction temperature induces homogeneous gas-phase reactions to form dehydrogenated and cyclized C2+ products. The integration of novel single atom catalysts for NMC initiation with homogeneous reactions in a microreactor (e.g., a catalytic wall reactor) will enable unprecedented NMC performance. The objectives of this project are to: 1) synthesize isolated single atoms of various metals in a silica matrix to prove universality of these catalysts in CH4 activation; 2) utilize a wide range of experimental and computational techniques to probe in situ and operando the surface and bulk structure/property of the NMC catalysts; 3) mechanistically understand the reaction network by an integrated experimental and computational effort to identify rigorously species, temperature, and kinetics; and 4) validate and scale-up synthesis of robust catalysts and reactors for efficient NMC of natural gas guided by validated process modeling. The proposed system is designed to run at single-pass CH4 conversion and C2+ yields of >25%, with > 90% C2+ selectivity, and a lifetime of >1000h.
Impact
While methane, the primary component of natural gas, is a source of energy and economic growth, it can also be an environmental concern. Recent developments in horizontal drilling as well as enhanced extraction methods have resulted in production of an estimated 62.4 trillion m3 of ‘stranded’, or uneconomic, natural gas. Associated gas is often flared or vented at remote oil production sites. Leaked, flared, and/or vented gas represents a lost opportunity and reduces domestic energy security. To address this problem, the University of Maryland College Park (UMD) and the University of Delaware (UD) propose to develop transformative isolated single atom metal/silica (M/SiO2) catalysts and an intensified catalytic wall reactor technology to convert CH4 to value-added C2+ and H2 products.
Accomplishments (most recent listed first)
- Systemic studies were completed on the synthesis of the metal/silica (M/SiO2) catalysts in which the metal type, composition, and silica support were varied. In particular, the metal type includes Fe, Cu, Co, Ni and Mn, which are abundant and low-cost materials in the earth’s crust. The composition of each metal was varied from 0.01wt% to 1.50wt%. The silica supports include zeolite based silicalite-1(S), quartz (Q), quartz melt (QM), and cristobalite (CRS).
- Two new approaches were used to prepare the catalyst with different composition and structures. The first approach is the furnace fusion that produces cristobalite-based catalysts. The second approach is H2/Air flame fusion with higher synthesis temperature than furnace fusion, which generates quartz and vitreous quartz-based catalysts. These two approaches are easy to operate but prepare catalysts with diverse structures and properties.
- Density functional theory (DFT) was used to compute ab initio phase diagram under methane conversion conditions. The computation predicts that FeC2 on silica is the thermodynamically stable catalyst species. A significant difference on the degree of carbonization of the metal in different metal/silica catalysts. DFT mapped out the surface reaction mechanism of methane coupling to C2 and C3 hydrocarbons on the FeC2 site.
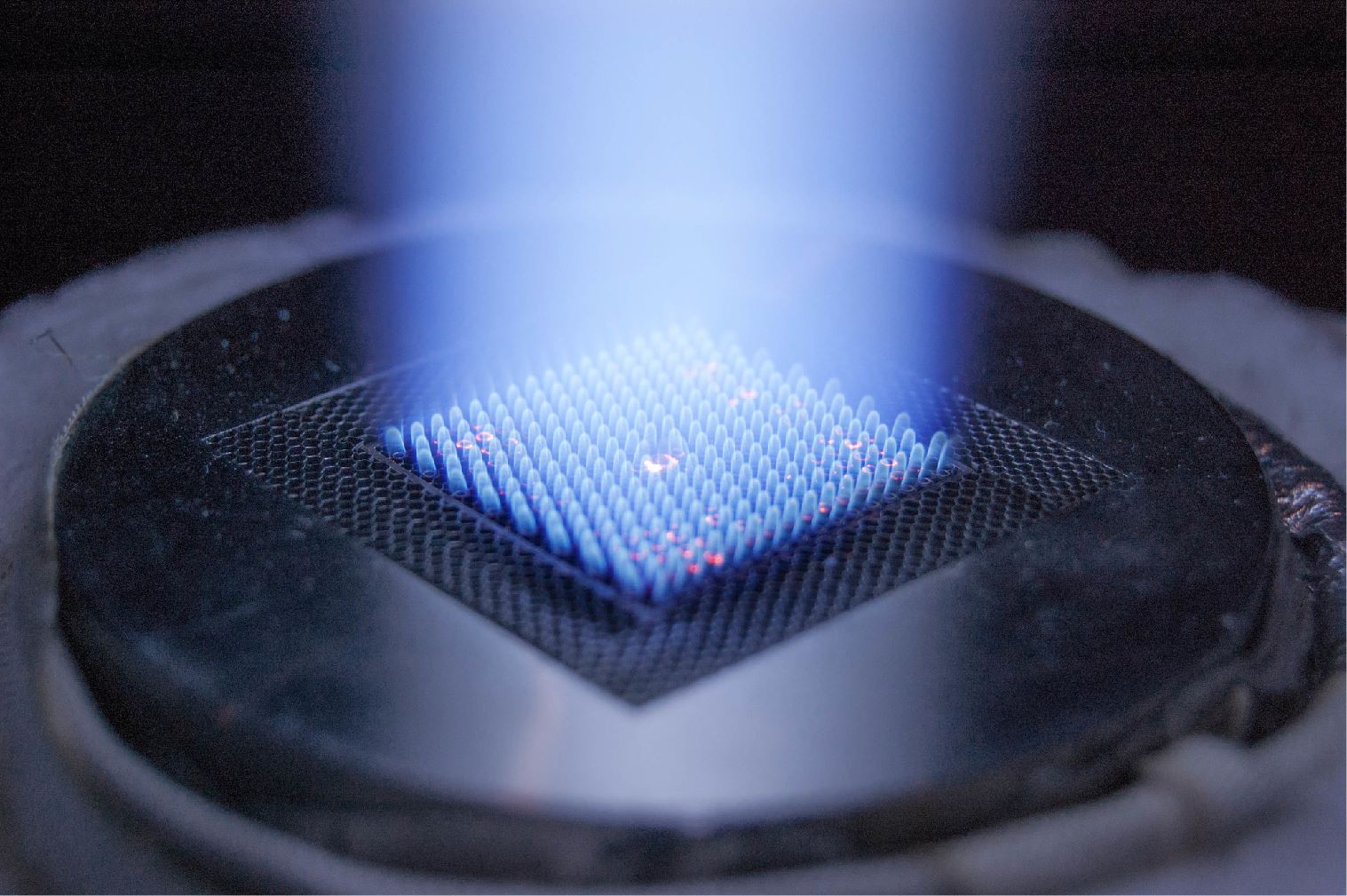
- An autothermal millisecond catalytic wall reactor was invented to resolve endothermicity of methane activation requiring high temperatures with subsequent promotion of heavy hydrocarbon and coke-forming reactions. Sharp temperature gradients along axial direction controlled by catalyst zoning in the reactor, together with short contact-time inhibited the heavy product or coke formation, thereby promoting catalyst stability. This reactor innovation led to unprecedented NMC performance with ~37% single-pass CH4 conversion, >99% C2+ (i.e., C2 and aromatics) selectivity, negligible coke formation, and autothermal self-sustainability. Process simulations studied temperature and species concentration profiles in the reactor.
- Extensive characterizations on the catalysts were conducted to understand their physicochemical properties. Among all the tested metal types, Cu, Co, and Ni can activate methane at lower temperatures, but lead to coke deposition. Mn is less active than Fe in the catalyst. Fe is still the best candidate among all the catalysts at the tested temperature range (900-1100 °C). The low metal concentration cannot activate methane to high conversions, while high metal concentration results in heavy aromatics formation and coke deposition. Among different SiO2 supports, vitreous silica supported Fe has the least aromatics and coke formation, and thus has the best stability.
Current Status
The team is conducting in-situ characterizations on the methane activation over the Fe/SiO2 catalyst in both fixed-bed and catalytic wall reactor settings in a customized capillary sampling system connected to a process mass spectrometer. The study will reveal the contributions from gas phase and surface reactions to the measured conversions. The catalytic performance of the catalysts with different compositions in the induction period of NMC are being studied. The studies for pushing high methane conversions in the catalytic wall reactor is ongoing. The computational calculations for optimizing catalyst and reactor operation conditions are being conducted.