As the nation and world strive to reduce emissions of carbon dioxide (CO2) associated with the energy ecosystem, NETL is leveraging its world-class expertise and facilities to drive innovation and deliver solutions. NETL researchers are designing and developing novel materials, devices and processes that will become a viable, affordable part of technologies to reduce greenhouse gas emissions.
As opposed to point-source carbon capture (such as capture from power plant or industrial plant emissions), direct air capture (DAC) is the process of capturing carbon directly from the atmosphere. DAC is envisioned as a process to help mitigate greenhouse gas emissions from technologies where point-source capture is difficult. DAC involves the capture of CO2 from air where it is present at only ~400 parts per million (ppm).
One of the largest challenges for DAC is finding materials that can realize the potential of the process. At NETL, researchers are using both experimental and computational methods to screen, design and synthesize DAC materials such as metal organic frameworks (MOFs), solid oxide materials, amine-impregnated polymer hollow fibers, and carbon-based nano porous sorbents.
“At NETL, we use experimental and computational methods in a cooperative way to accelerate the discovery and maturation of highly successful carbon capture materials,” said Jan Steckel, computational scientist and principal investigator on a number of projects for the development of capture materials.
For example, MOF materials show promise for carbon capture due to their high surface area, capacity for high sorption selectivity, and unique ability to tailor the properties for the desired application. The objective in studying MOF materials is to develop computational methods coupled with NETL’s world-class high-performance computer Joule to screen gas sorption and diffusion in flexible MOF materials across the rather large space these materials represent. If CO2 adsorption is linked to a structural change in the MOF, it could be possible to leverage this feature to reduce the energy input required to collect adsorbed CO2 and regenerate the sorbent. The desired material characteristics of this work are identification of MOFs with high CO2 loading at low pressure, high CO2/nitrogen selectivity, and low regeneration energies.
Solid oxide sorbents are also promising candidates for CO2 sorbent applications through a reversible chemical transformation due to their high CO2 absorption capacities at low CO2 pressure. High adsorption capacity at low CO2 pressure coupled with very low cost makes this class of sorbent particularly attractive for DAC applications. However, a single material normally cannot satisfy the technology needs, which has prevented the use of solid sorbents for carbon capture. To address this R&D challenge, NETL has focused on mixing different types of elements to make mixed and doped solid sorbents that can meet the required needs. Upon design and synthesis of the materials, NETL leverages unique laboratory capabilities to elucidate their performance at conditions consistent with application
NETL researchers have previously created basic immobilized amine sorbents (BIAS) with outstanding performance that offers superior resistance to amine leaching, which has been recognized through a number of awards including the prestigious R&D 100. Recently, NETL researchers recognized many advantages could be gained by incorporating features of the BIAS sorbent into a hollow fiber. To that end, NETL researchers are refining BIAS sorbents into a hollow chemisorption fiber sorbent using unique laboratory capabilities to synthesis tailored fibers from historic work on membrane development. The hollow fibers offer high mechanical strength, low pressure drop, and efficient heat transfer for ambient air carbon capture.
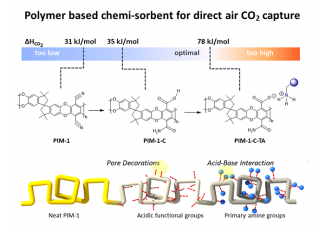
Another sorbent technology developed recently is porous polymeric sorbents (PIMs). The PIM sorbent material concept is presented in Figure 1. This material is based on a highly porous polymer known as PIM-1. NETL researchers chemically convert functional groups on the PIM-1 polymer into acidic groups (labeled PIM-1-C in the figure). These acidic functional groups are subsequently reacted with amine molecules, forming the immobilized amine sorbent (labeled PIM-1-C-TA in the figure). Moreover, sorbents can be directly processed into hollow fibers, pellets etc., which provide high CO2 capture performance.
NETL is a U.S. Department of Energy national laboratory that drives innovation and delivers technological solutions for an environmentally sustainable and prosperous energy future. By leveraging its world-class talent and research facilities, NETL is ensuring affordable, abundant and reliable energy that drives a robust economy and national security, while developing technologies to manage carbon across the full life cycle, enabling environmental sustainability for all Americans.